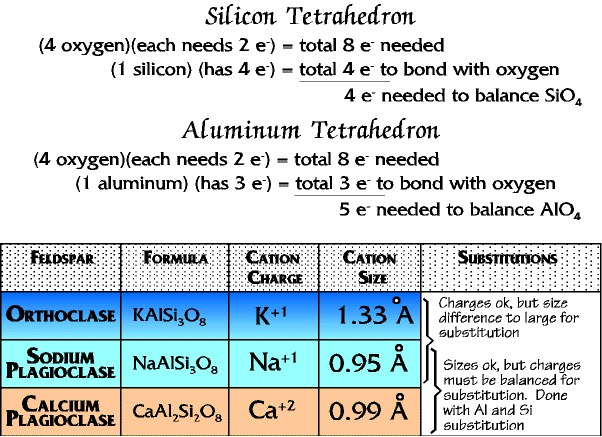
Feldspars
Feldspars substitute some alumina tetrahedra for silica tetrahedra. This results in a charge imbalance, however, since the alumina tetrahedra is a -5 anion, and the silica tetrahedron is a -4 anion. To balance the charge feldspars add metallic cations, one positive charge for each alumina tetrahedron. The available cations are calcium (+2), sodium (+1), and potassium (+1). Each calcium requires two alumina tetrahedra to balance, and each sodium and potassium one alumina tetrahedra, so it might seem reasonable that the sodium and potassium could substitute for each other. But there is also a size balance necessary, and it is more important. So, since calcium and sodium are nearly the same size they substitute for each other in a solid solution series for the plagioclases, each calcium adding two alumina tetrahedra to the crystal, and each sodium one alumina tetrahedra.
And since potassium is much larger it goes by itself to form orthoclase.