Hardness and Acid Reaction Tests
For Minerals and Rocks
For Minerals and Rocks
The heart of science is the ability to make accurate observations about the natural world. Two of the most common properties we measure in mineral and rock identification are hardness and reaction with dilute hydrochloric acid. It is very difficult to avoid these two tests. Some guidelines and suggestions are below
HARDNESS:
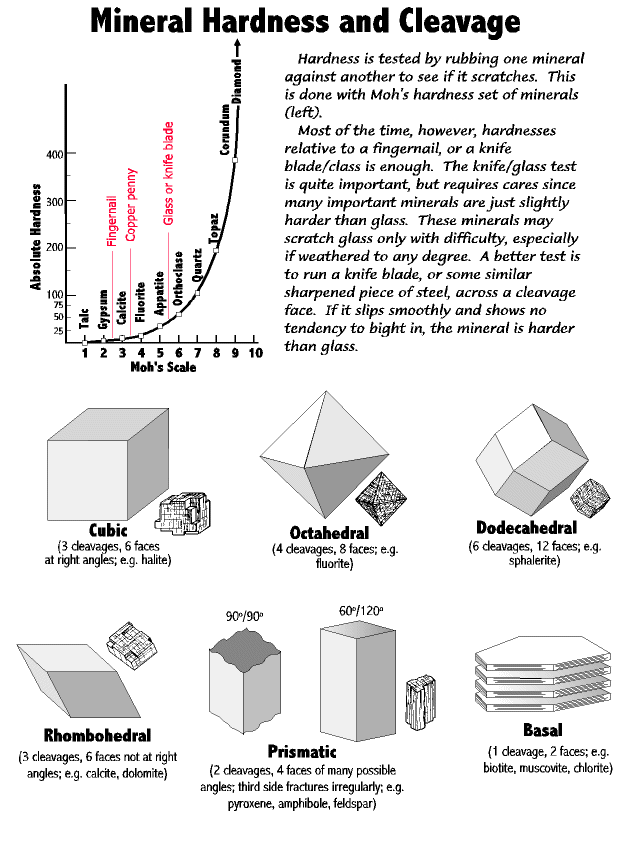 The technical tool is the Moh's hardness scale which divide minerals and rocks into ten hardness steps. Practically, though, we can get away most of the time with harder or softer than glass, and harder or softer than a fingernail (click image for pdf file). For the glass hardness test we use glass plates about 2 inches square, purchased from any hardware or glass supplier. Students place the glass on a hard, flat surface, and then firmly run the specimen across the glass - sometimes several times to make sure the test is accurate.
The technical tool is the Moh's hardness scale which divide minerals and rocks into ten hardness steps. Practically, though, we can get away most of the time with harder or softer than glass, and harder or softer than a fingernail (click image for pdf file). For the glass hardness test we use glass plates about 2 inches square, purchased from any hardware or glass supplier. Students place the glass on a hard, flat surface, and then firmly run the specimen across the glass - sometimes several times to make sure the test is accurate.The cautions are: One, sometimes a specimen will rub off on the glass rather than actually scratching it. Run a fingernail across the glass to feel for a nick.
Two, some specimens softer than glass are contaminated with things harder than glass and will give a false test. Rub several times in several places to be sure.
Three, many important minerals are just slightly harder than glass, so it takes care to do a good hardness test. (And frustratingly, if the specimen is weathered it will give a false test.)
HCl ACID REACTION:
Carbonate minerals and rocks (i.e. those that have calcite and/or dolomite in them, such as limestones, dolostone, and marble) are very common, and the quickest way to identify them is with an acid reaction.
The acid used is dilute hydrochloric acid. Just a few percent solution is good enough, and never more than 10 percent. We keep it in a squeeze or dropper bottle so that just a drop at a time can be placed on the specimen. And we wash the specimen well each time. Acid this weak will not hurt you, but it will eat holes in your clothes.
Any rock with calcite reacts quickly with large bubbles. Dolomite, however, reacts very slowly, and pure dolomite must be powdered to get even a weak reaction, very fine, slow bubbles. Calcite and dolomite are a solid solution series; that is, there is a complete intergradation from 100% of one to 100% of the other, so in between there are intermediate reactions.
The biggest caution, aside from safety, is that many non-carbonate rocks are contaminated with more or less small amounts of calcite. These contaminated rocks react just as well as pure carbonate rocks, sometimes better. It takes careful observation to be sure, but look for a residue that did not react with the acid. This is a good indication that something else is in the rock, or may even be the majority of the rock.
Return to:
Other pages associated with this page:
Go on to more detailed pages